
Properties and Overview of Gadolinium
Overview:
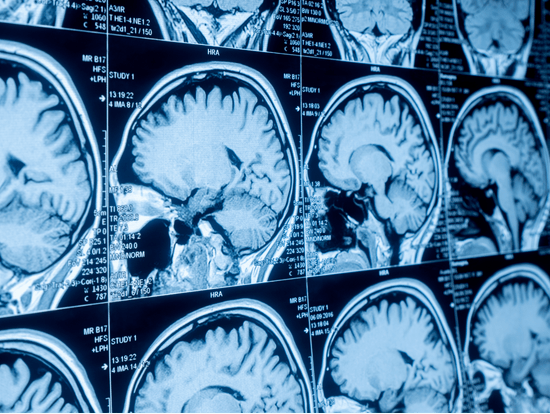 Gadolinium (Gd) a chemical element with the symbol Gd and atomic number 64, holds a significant place in the history of chemistry. Discovered in 1880 by the Swiss chemist Jean Charles Galissard de Marignac, it was identified in the mineral samarskite. The element is named after the Finnish chemist Johan Gadolin, one of the pioneering researchers of rare earth elements. This historical context adds depth to our understanding of gadolinium's unique properties and applications. Physically, gadolinium is a relatively soft metal with a bright, silvery appearance. It has a melting point of 1,312°C and a boiling point of 3,233°C. The metal is relatively stable in dry air but tarnishes in moist air, forming a thin oxide layer on its surface. Gadolinium has a hexagonal close-packed crystal structure at room temperature, which changes to a body-centered cubic structure when heated above 1,235°C. It has a density of about 7.9g/cm3. One of gadolinium's most notable physical properties is its high magnetic susceptibility, making it one of the most magnetic elements. Gadolinium exhibits ferromagnetic properties at temperatures below 20°C but becomes paramagnetic at higher temperatures. This unique magnetic behavior, the Curie point, is helpful in various technological applications.
Gadolinium (Gd) a chemical element with the symbol Gd and atomic number 64, holds a significant place in the history of chemistry. Discovered in 1880 by the Swiss chemist Jean Charles Galissard de Marignac, it was identified in the mineral samarskite. The element is named after the Finnish chemist Johan Gadolin, one of the pioneering researchers of rare earth elements. This historical context adds depth to our understanding of gadolinium's unique properties and applications. Physically, gadolinium is a relatively soft metal with a bright, silvery appearance. It has a melting point of 1,312°C and a boiling point of 3,233°C. The metal is relatively stable in dry air but tarnishes in moist air, forming a thin oxide layer on its surface. Gadolinium has a hexagonal close-packed crystal structure at room temperature, which changes to a body-centered cubic structure when heated above 1,235°C. It has a density of about 7.9g/cm3. One of gadolinium's most notable physical properties is its high magnetic susceptibility, making it one of the most magnetic elements. Gadolinium exhibits ferromagnetic properties at temperatures below 20°C but becomes paramagnetic at higher temperatures. This unique magnetic behavior, the Curie point, is helpful in various technological applications.
Chemically, gadolinium is relatively reactive for a rare earth metal. It reacts slowly with oxygen, forming gadolinium oxide (Gd2O3), a white, powdery substance that is insoluble in water but soluble in mineral acids. Gadolinium reacts with water at room temperature to form gadolinium hydroxide and hydrogen gas. It also reacts readily with acids, forming various gadolinium salts. The element commonly exhibits a +3 oxidation state in its compounds, which include gadolinium chloride (GdCl3), gadolinium nitrate (Gd(NO3)3), and gadolinium sulfate (Gd2(SO4)3). These compounds are typically colorless or white and are used in various chemical processes and applications. Gadolinium's chemistry is characterized by unpaired f-electrons, contributing to its unique electronic and magnetic properties.
Regarding safety, gadolinium and its compounds must be handled carefully, especially in medical and industrial applications. While gadolinium metal is not particularly toxic, soluble gadolinium salts can be harmful if ingested or inhaled, as they can irritate the skin, eyes, and respiratory tract. There is also some concern about the long-term effects of gadolinium exposure in patients who undergo MRI scans with gadolinium-based contrast agents, particularly in individuals with impaired kidney function. These concerns have led to developing newer, safer gadolinium compounds with reduced toxicity. In industrial settings, proper safety measures, such as personal protective equipment (PPE), ventilation, and proper storage, are essential to minimize the risk of exposure to gadolinium dust or fumes.
Production:
The production of gadolinium involves extracting the element from minerals such as monazite and bastnaesite, rich sources of rare earth elements. These minerals are mined in countries with significant rare earth deposits, including China, the United States, India, and Australia. The extraction process begins with the concentration of these minerals, followed by the separation of rare earth elements through solvent extraction and ion-exchange chromatography. After separation, gadolinium is converted to its oxide or chloride form, which can then be reduced to metallic gadolinium using calcium or other reducing agents in a high-temperature process. The production of gadolinium requires specialized techniques and facilities, given the complexity of separating it from other rare earth elements with similar chemical properties.
Applications:
Gadolinium has several critical applications in various fields due to its unique magnetic and neutron absorption properties. One of the most significant uses of gadolinium is in the medical field, particularly in magnetic resonance imaging (MRI). Gadolinium compounds, such as gadolinium chelates, are used as contrast agents in MRI scans to enhance the quality of the images. These compounds are injected into the bloodstream, where they improve the contrast of tissues and blood vessels, allowing for more precise and detailed imaging of the body's internal structures. Gadolinium is also used to produce phosphors for color television tubes and to manufacture compact discs and computer memory devices. In the nuclear industry, gadolinium is used as a neutron absorber in control rods and shielding materials due to its high neutron capture cross-section, which helps control the rate of nuclear reactions in reactors.
Summary:
Gadolinium is not just a valuable rare earth element, but also a catalyst for future research and innovation. Its unique physical and chemical properties, particularly its magnetic and neutron absorption capabilities, have paved the way for its significant role in medical imaging, electronics, and nuclear technology. However, the challenges associated with its production, reactivity, and potential toxicity necessitate careful handling and management to ensure safe and sustainable use. As technology continues to evolve, the demand for gadolinium and other rare earth elements is expected to grow, driving ongoing research into more efficient extraction methods and alternative applications. This potential for growth and innovation should inspire us all.
See a comprehensive list of atomic, electrical, mechanical, physical and thermal properties for gadolinium below:
Atomic Structure of Gadolinium

Unfamiliar with a property? Click it's description to be given a full definition in the GLOSSARY
Require different units not displayed?
CONVERT VARIOUS UNITS HERE
Atomic Properties of Gadolinium
| Atomic Property (Units) | Value |
|---|---|
| Gadolinium Atomic Electron Configuration | [Xe] 4f75d16s2 |
| Gadolinium Atomic Mass (amu) | 157.25 |
| Gadolinium Atomic Number | 64 |
| Gadolinium Chemical Element Symbol | Gd |
| Gadolinium Covalent Radius (Å) | 1.96 |
| Gadolinium Crystal Structure | Hexagonal Close-Packed (HCP) |
| Gadolinium Electronegativity (Pauling Scale) | 1.2 |
| Gadolinium Electrons per Orbital Shell (inner most first) | 2, 8, 18, 25, 9, 2 |
| Gadolinium Half-Life (Years) | N/A - Stable |
| Gadolinium Lattice Parameter / Lattice Constant (Å) | a = 3.63, c = 5.78 |
| Gadolinium Number of Electron Orbital Shells | 6 |
| Gadolinium Number of Electrons | 64 |
| Gadolinium Number of Neutrons | 64 |
| Gadolinium Number of Protons | 64 |
| Gadolinium Periodic Table Series | Lanthanides |
| Gadolinium Phase at 'Standard Temperature and Pressure' | Solid |
| Gadolinium Stable Isotopes | Gd-157, Gd-158 |
Unfamiliar with a property? Click it's description to be given a full definition in the GLOSSARY
Require different units not displayed?
CONVERT VARIOUS UNITS HERE
Electrical Properties of Gadolinium
| Electrical Property (Units) | Value |
|---|---|
| Gadolinium Dielectric Constant at 'Standard Temperature and Pressure' | Unknown |
| Gadolinium Electrical Breakdown Voltage at Atmospheric Pressure (kV/mm) | Unknown |
| Gadolinium Electrical Conductivity (S/m) | 7.4E+05 |
| Gadolinium Electrical Resistivity at Room Temperature (25°C) (Ω·m) | 1.351E-06 |
| Gadolinium Magnetic Property | Ferromagnetic |
| Gadolinium Superconducting Transition Temperature (K) | N/A - Not a Super Conductor |
| Gadolinium Temperature Coefficient of Resistance (°C⁻¹) | +0.0045 (4500 ppm/°C) |
Unfamiliar with a property? Click it's description to be given a full definition in the GLOSSARY
Require different units not displayed?
CONVERT VARIOUS UNITS HERE
Mechanical Properties of Gadolinium
| Mechanical Property (Units) | Value |
|---|---|
| Gadolinium Compressive Strength (MPa) | 180 |
| Gadolinium Ductile to Brittle Transition Temperature (°C) | None (Ductile Always) |
| Gadolinium Fatigue Limit (MPa) | Unknown |
| Gadolinium Fracture Toughness (MPa·√m) | 4 |
| Gadolinium Hardness Brinell | 65 |
| Gadolinium Hardness Rockwell | 30 (HRA) |
| Gadolinium Hardness Vickers | 570 |
| Gadolinium Heat Deflection Temperature (°C) | N/A - Not a Polymer |
| Gadolinium Modulus of Elasticity / Young's Modulus (GPa) | 54.8 |
| Gadolinium Percent Elongation (%) | 25 |
| Gadolinium Poissons Ratio | 0.26 |
| Gadolinium Shear Modulus (GPa) | 21.8 |
| Gadolinium Shear Strength (MPa) | 130 |
| Gadolinium Ultimate Tensile Strength (MPa) | 210 |
| Gadolinium Yield Strength (MPa) | 210 |
Unfamiliar with a property? Click it's description to be given a full definition in the GLOSSARY
Require different units not displayed?
CONVERT VARIOUS UNITS HERE
Physical Properties of Gadolinium
Unfamiliar with a property? Click it's description to be given a full definition in the GLOSSARY
Require different units not displayed?
CONVERT VARIOUS UNITS HERE
Thermal Properties of Gadolinium
| Thermal Property (Units) | Value |
|---|---|
| Gadolinium Coefficient of Thermal Expansion (µm/m·K) | 9.4 |
| Gadolinium Emissivity Coefficient | Unknown |
| Gadolinium Specific Heat Capacity (J/kg·K) | 236 |
| Gadolinium Thermal Conductivity (W/m.K) | 10.6 |
| Gadolinium Thermal Conductivity (BTU/h·ft·°F) | 6.13 |
Unfamiliar with a property? Click it's description to be given a full definition in the GLOSSARY
Require different units not displayed?
CONVERT VARIOUS UNITS HERE
 ADDED TO MY FAVORITES!
ADDED TO MY FAVORITES! REMOVED FROM MY FAVORITES!
REMOVED FROM MY FAVORITES!